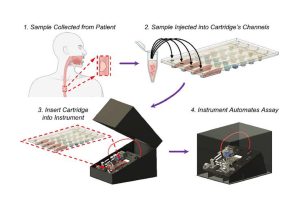
Esophageal cancer carries a grim prognosis even when the best medical care is available. In the developing world, where access to medical resources is limited, it is essentially a death sentence. But Johns Hopkins researchers have developed a system aimed at improving the rate of survival for those with this deadly cancer—particularly those in less-developed areas—through earlier diagnosis.
Their Automated Cartridge-based Cancer Early Screening System (ACCESS) is an inexpensive, shoebox-sized platform that can detect esophageal cancer biomarkers from a small bit of the patient’s DNA. Simply place the sample on a credit-card-sized cartridge, then insert it into the device, which autonomously screens it for molecular signatures associated with the disease. The system is described in ACS Nano.

Tza-Huei (Jeff) Wang
“We recognized that a promising solution for early detection of esophageal cancer lies in an easy-to-use testing platform, like those used for COVID antigen testing, that would be accessible to regions with limited healthcare resources,” said Alex Hasnain, PhD student in biomedical engineering, who worked with a team led by Tza-Huei (Jeff) Wang, a professor of mechanical engineering at Johns Hopkins Whiting School of Engineering, on the project.
One of the most common and deadly cancers, esophageal cancer has a survival rate of 20% in developed countries and less than 5% in developing countries. Current detection methods—radiology, endoscopy, and biopsy—often are not performed until the disease is too far advanced for anything but palliative treatment, according to Wang.
“Conventional detection methods for esophageal cancer require sedation, are invasive, are out of reach to many patients in need, and, most importantly, often detect the disease too late,” Wang said. “This is a critical problem in need of novel biomedical solutions, especially in parts of the world with limited healthcare resources.”
ACCESS uses a series of steps to analyze patient DNA samples, which the team hopes will someday be taken by a simple, noninvasive sponge swab to the inside of the patient’s throat. For testing purposes during the development of the device, the researchers worked with Stephen Meltzer, professor of medicine and oncology at Johns Hopkins University School of Medicine, to use samples from biopsies taken from patients diagnosed previously with esophageal cancer and a healthy control group.
First, the patient’s cells are mixed with silica magnetic beads, a bisulfite conversion solution, and thermolabile proteinase K (an enzyme that breaks down proteins), and injected into the disposable cartridge’s four independent channels. The cartridge is then inserted into the portable instrument, which autonomously searches for the esophageal cancer pathogen’s signature genetic markers. Several hours later, a diagnosis is available.
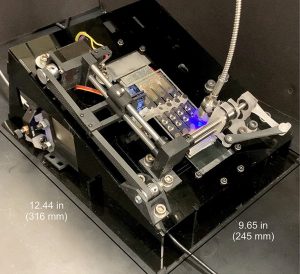
“We showed that ACCESS can simultaneously detect multiple methylation biomarkers from esophageal squamous cell carcinoma (ESCC) tissue specimens obtained from patients,” Hasnain said. “ACCESS can also correctly detect when there is no such methylation biomarkers present. These results, along with the platform’s full automation, illustrate that ACCESS not only simplifies the detection for methylation biomarkers but also has the potential for future clinical use.”
The team is currently refining the design of the cartridge and instrument to make it easier to use, and developing the minimally-invasive sponge-like tool that will be used to collect esophageal tissue samples for testing. ACCESS will also be brought for testing to a clinic in Uganda, in collaboration with Johns Hopkins for on-site studies.
“We anticipate that an improved version of ACCESS will become a valuable tool for early cancer detection, especially in areas with limited medical resources,” Hasnain said. “Additionally, our magnetic-based DNA extraction has proven effective across various different sample types, and the nucleic acid test can be modified for different methylation biomarkers. So we expect that ACCESS can also be applied to eventually detect other cancer types, as well.”