Tear Formation and Propagation
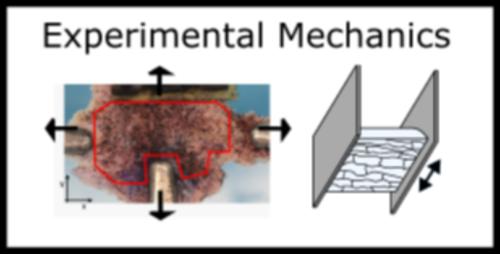
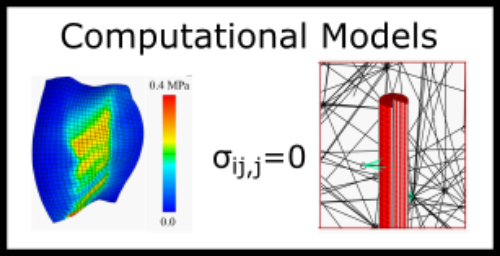
The cause of tears in some musculoskeletal tissues such as the meniscus after an ACL tear or another traumatic event are known. However, the formation of tears in some musculoskeletal tissues including the intervertebral disc are unknown. Most of these tissues are heterogeneous and their structure and mechanics change with age and disease. These factors in addition to the fact that these tears occur under a multiaxial load state make understanding tear formation and propagation more challenging. By implementing a unique combination of experimental and computational approaches, we are able to further evaluate the failure thresholds and mechanisms that fibrous soft musculoskeletal tissues undergo. This work will lead to further understanding of who and what motions make some people susceptible to failure and tears in their musculoskeletal tissues.
Facet Joint Mechanics and the Spine
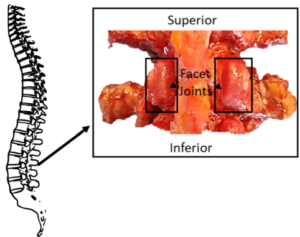
The facet joint in the spine is a known source of back pain, but identifying when the source of back pain is the facet joint or another portion of the spine is difficult. This difficult arises from the fact that arthritis in the facet joints occurs at similar rates to degeneration of the intervertebral discs. Therefore, by studying the factors that lead to facet joint degeneration and inflammation, we are advancing understanding of the degeneration process of the facet joints and the spinal motion segment that could lead to pain.
Discrete Fiber Network Modeling and Mechanotransduction
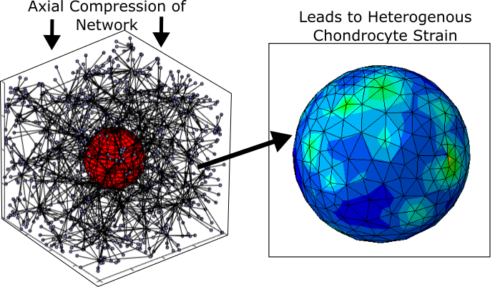
Mechanotransductive signaling of a cell will depend on the stress and strain a cell experiences due to joint scale loading, which can depend on the local tissue environment, tissue heterogeneity, and more. However, many mechanobiological studies of cells occur with a set of isolated cells in a dish or a hydrogel. To translate these results to native tissues and predict the signaling that may occur in-vivo we have developed a discrete fiber network modeling approach. By coupling these models with mechanotransduction experiments and joint scale modeling, we will be able to predict and potentially prevent damage to cells. This approach and will also be applied to understand pain signaling by axons and the production of new matrix in engineered tissues.
Integration of Native and Engineered Tissues
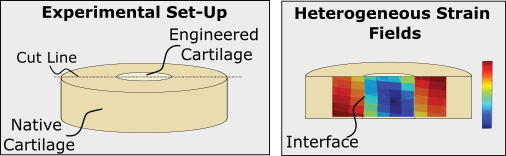
A major challenge in developing and advancing tissue engineering strategies includes understanding the integration strength between native and engineered tissue. This integration strength can be more difficult to understand due to the types of loads placed on the joint and variability in the implantation technique. Additionally, growth of the engineered construct may change the integration strength. Therefore, we aim to use a advanced mechanics techniques to understand which tissue engineering strategies and growth techniques allow for the best integration. We aim to apply this process to understand many engineered constructs including tissue engineered cartilage.

