The Role of Cornea and Sclera Biomechanics in Glaucoma
PI: Professor Thao (Vicky) Nguyen
Department of Mechanical Engineering
Lab: 216A Latrobe
Motivation
Glaucoma is a leading cause of blindness in the United States, and the second leading cause worldwide. Both epidemiological and laboratory studies show that the pathogenesis of glaucomatous optic nerve damage includes a strong association between the level of intraocular pressure (IOP) and glaucomatous damage to retinal ganglion cells (RGC), the neurons injured in this disease. There is consensus that the level of IOP generates a biomechanical response in the ocular tissues that is fundamental to the early events in glaucoma damage. The IOP is translated into mechanical stresses on ocular structures by the response of the eye wall, consisting of the cornea and sclera. The physical interaction of the cornea-sclera shell at the ONH is central to initial events in glaucoma damage, and improved knowledge of the biomechanical behavior of these structures will advance understanding of glaucoma injury, its early diagnosis, and prevention of the blindness caused by the disorder.
Protocol
We will characterize the mechanical deformation of the cornea and sclera of normal and glaucoma eye bank donor eyes in response to inflation, using in vitro digital image analysis. From the results, we will construct a microstructure based constitutive model for the anisotropic, nonlinear viscoelastic behavior of the cornea and sclera. Two collaborators will participate, Harry Quigley, MD, and Russell McCally, PhD, from the Wilmer Eye Institute, Johns Hopkins. Dr. Quigley is a leading investigator in the pathogenesis of glaucoma, and Dr. McCally is an expert in the anatomy and properties of ocular connective tissues. Specifically, the experimental protocol will be:
1) We will obtain cornea and sclera from normal and glaucoma eye-bank donor eyes. The eyes will be delivered from the Maryland eye-bank via professional courier.
2) The eyes will be dissected and tested immediately upon arrival to the lab.
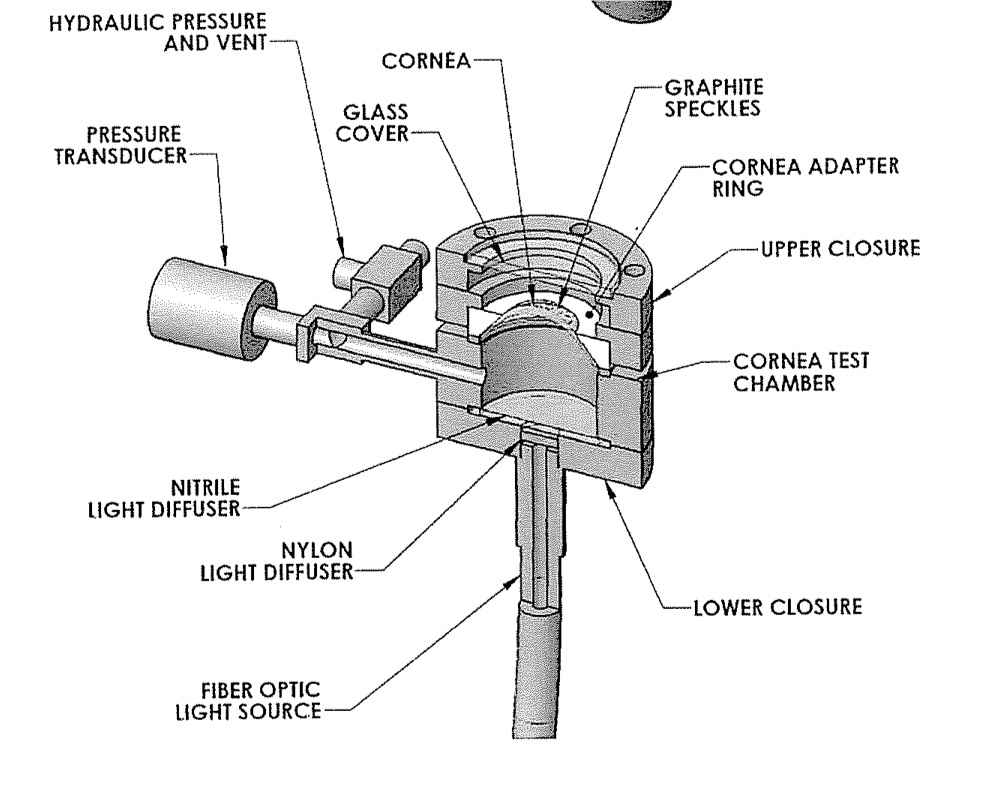
3) In the test, the cornea will be attached by a sclera rim, a few millimeters wide, to the inflation chamber. The sclera will be mounted by its more anterior portion and the posterior sclera will be tested in the same manner. The specimen will be inflated by an infusion of a balanced saline solution (BSS Plus) into the inflation chamber, which will be maintained at 37oC.
Potential Hazards
The potential hazards for this research project are connected with the handling and testing of hydrated human soft tissue. Human cornea and sclera will be tested using standard mechanical inflation and tension protocols. All testing equipment and preparation instruments will be considered contaminated; therefore an exposure risk is inherent in the experimental procedures. Preparation equipment will include the use of sharps (i.e. surgical scalpels) therefore proper training is required for all lab personnel. Lastly, inflating human tissue may increase the risk of spilling or splattering tissue fluids. Therefore, appropriate personal protective equipment will be supplied.
Safety Practices and Methods
BSL2 practices will be observed for this lab because human tissue will be tested. Appropriate biosafety training will be provided to all students and staff who work in the lab. All personnel will be aware of the safety procedures that are specific to the lab and its location. Emergency contact information and hazardous chemical spill information will be posted. Personal protection equipment such as lab coats, nitrile gloves, and safety glasses will also be provided to all who enter. For personnel involved with dissecting the tissue, N95 surgical masks will be provided. All contaminated surfaces and instruments will be cleaned with a 1:10 dilution of bleach, freshly prepared, after each experiment. Contaminated surfaces will be wiped down and instruments will be soaked for 20 minutes. All waste generated from the experiments will be disposed in the marked biohazard burn boxes, and all sharps (i.e. broken glass, scalpel blades, etc.) will be disposed in marked biohazard sharps containers. No food or beverages will be allowed in the lab.
Because of the risk of bursting during the mechanical inflation test, care must be taken to avoid exposure from splash. Testing pressures will range in the range of 1-5psi. These pressures are well within the working range of the equipment and strength of the eye tissue therefore keeping the bursting risk low. Regardless of the low working pressure, the design of the inflation apparatus greatly reduces the risk of exposure. The mechanical testing fixture was designed to provide shielding from potential bursts and spills. See Figure 1 for fixture design.
In the instance of the specimen bursting, the glass window will prevent fluid from being aerosolized into the general lab space. Another potential site of rupture exists within the tubing and syringe system of the testing setup. To further reduce the risk of splash in the instance of a system rupture, we will use luer lock tube fittings that can withstand up to 99 psi of pressure.
Spills
In the instance of a biological spill, the spill will be immediately contained by surrounding and covering the spill with a 1:10 dilution of bleach, freshly prepared. The bleach should remain on the spill for at least 30 minutes. Then it should be cleaned up with a paper towel and discarded in the biological solids hazardous waste receptacle.
Biological Exposure
In the case of biological exposure, the exposed worker will need to call Occupational Health IMMEDIATELY (410-516-0450) or Security during off-hours (410-516-7777). The healthcare provider will manage the patient according to the Hopkins protocol. Please refer Johns Hopkins Safety Manuel for further details.
Figure 1: Eye inflation apparatus.